38 lewis dot diagram for krypton
Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Lewis (Dot) Diagram: Even atoms with more than 20 electrons are easy. Example 4: Krypton Krypton is in group 8A. Krypton atoms have 8 valence electrons. Ra Ra Radium 88 Lewis (Dot) Diagram: Example 5: Radium Radium is in group 2A. The Lewis dot-diagram was created by Gilbert N. Lewis. In 1916, he showcased it in an article called The Atom and the Molecule. [15] For example, the nitrogen atom has 5 valence electrons, so this is what the Lewis dot-diagram would look like: Nitrogen = a valence electron. Figure 5. A Lewis dot diagram of nitrogen. Summary of the Diagrams
Krypton. Lewis (Dot) Diagram: Even atoms with more than. 20 electrons are easy. The electron-dot structure can be used for any elements. It just shows how electrons are distributed between bonds or number of valence. The electron-dot structure can be used for any elements. It just shows how electrons are distributed between bonds or number of ...

Lewis dot diagram for krypton
Krypton. Lewis (Dot) Diagram: Even atoms with more than. 20 electrons are easy.Krypton difluoride, or KrF 2, has the Lewis structure of a krypton atom with 3 lone pairs, single bonded to two fluorine atoms, each also containing 3 lone pairs. Krypton has 8 valence electrons, whereas fluorine contains 7 valence electrons. A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ... Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1.
Lewis dot diagram for krypton. (It does not matter what order the positions are used.) For example, the Lewis electron dot diagram for calcium is simply. A Lewis structure of calcium is shown ... A step-by-step explanation of how to draw the KrBr4 Lewis Dot Structure.For the KrBr4 structure use the periodic table to find the total number of valence el... A step-by-step explanation of how to draw the KrF4 Lewis Dot Structure (Krypton Tetrafluoride)For the KrF4 Lewis structure, calculate the total number of val... A step-by-step explanation of how to draw the KrCl4 Lewis Dot Structure.For the KrCl4 structure use the periodic table to find the total number of valence el...
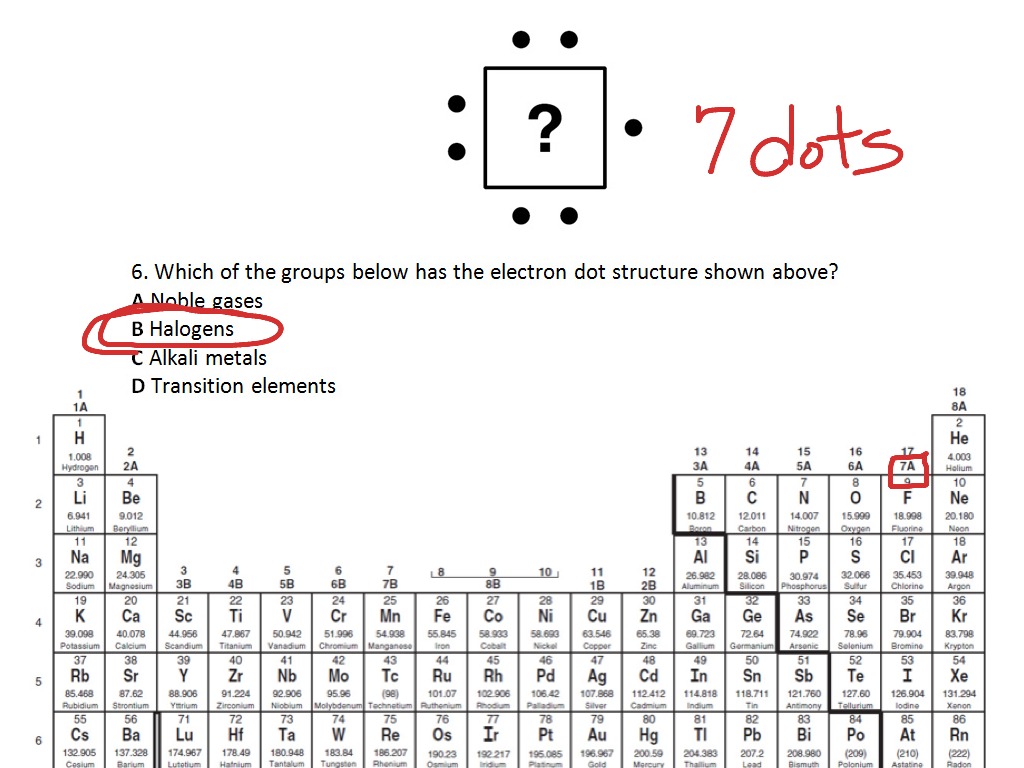
Bohr Model Lewis dot diagram. 1. Determine the element's symbol 2. Determine the number of electrons 3. Determine number of valence electrons 4. Now draw your ... Krypton 84.80 Xenon 131.29 Radon 222.018 118 Uuo unknown VilB Manganese 54.938 Technetium 98.907 Re Rhenium 186.207 107 [264] Iron 55.933 Ruthenium 101.07 os 4 A compound has a mass of The number of significant figures in this mass is — F 2 G 4 H 5 J 7 2 6632 10. × 2 g/mol. 3 Which of the following is the correct Lewis electron-dot diagram for the Jan 28, 2021 — You can here understand the interaction and the actual numbers of Krypton valence electrons. We typically use the Lewis dot diagram to unfold ... Krypton has a full outer shell of eight electrons, so it would beeight dots, either around the outer circle if you're doing a fullcircle diagram, or just two dots above, below , to the left, and tothe right of the Kr. Krypton 36 Lewis (Dot) Diagram: Even atoms with more than 20 electrons are easy. Example 4: Krypton Krypton is in group 8A.
Dec 14, 2019 — Explanation: Kripton (Kr) is a noble gas found in the 18th group of the periodic table. In the electron dot diagram we show the electrons in the ...1 answer · 4 votes: You may find the dot diagram for kripton (Kr) in the attached picture.Explanation:Kripton (Kr) is a noble gas found in the 18th group of the periodic table. ... Krypton Electron Dot Diagram. Krypton has a full outer shell of eight electrons, so it would beeight dots, either around the outer circle if you're doing a fullcircle diagram, or just two dots above, . Since it is in Group 8A it will have 8 valence electrons. When you draw the Lewis structure for Krypton you'll put eight "dots" or valance. Krypton 36 Lewis (Dot) Diagram: Even atoms with more than 20 electrons are easy. Example 4: Krypton Krypton is in group 8A. Krypton atoms have 8 valence electrons. Ra Ra Radium 88 Lewis (Dot) Diagram: Example 5: Radium Radium is in group 2A. Radium atoms have 2 valence electrons. Ra Ra Radium 88 A Lewis electron dot diagram A representation of the valence electrons of an atom that uses dots around the symbol of the element. (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element.
Atoms and Atomic Structure. What is Lewis dot diagram for krypton? Wiki User. ∙ 2013-10-23 00:30:55
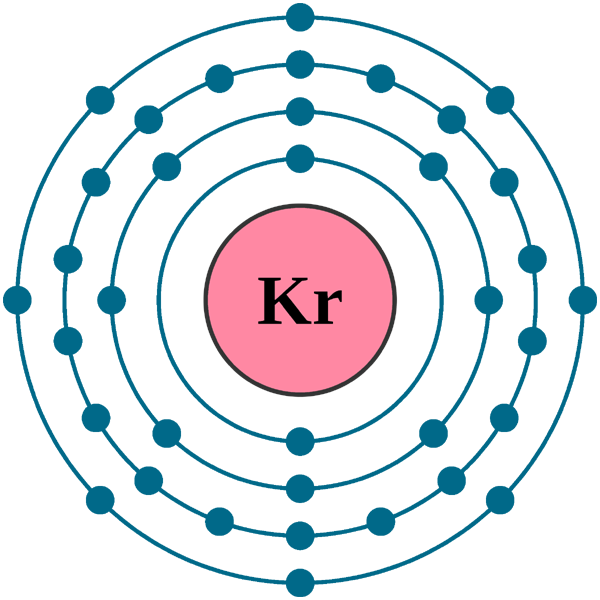
Atomic Structure of Krypton ... Electron Configuration: 1s2 2s2p6 3s2p6d10 4s2p6.
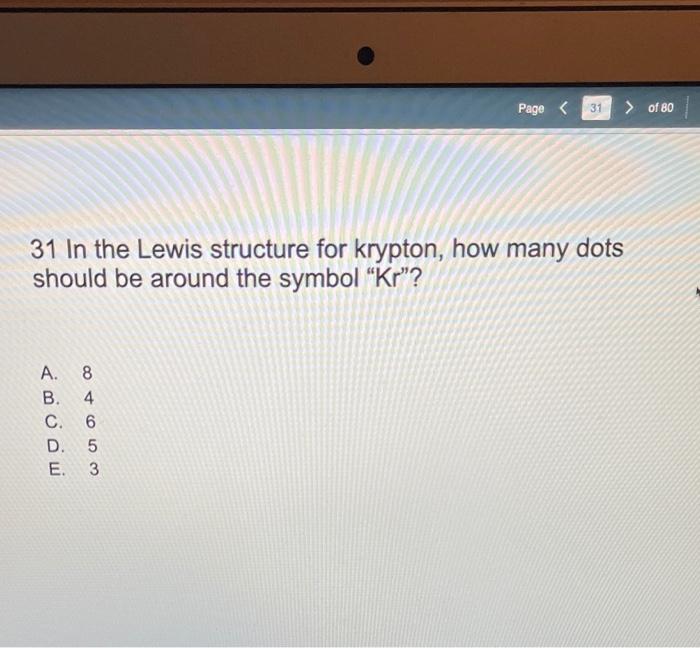
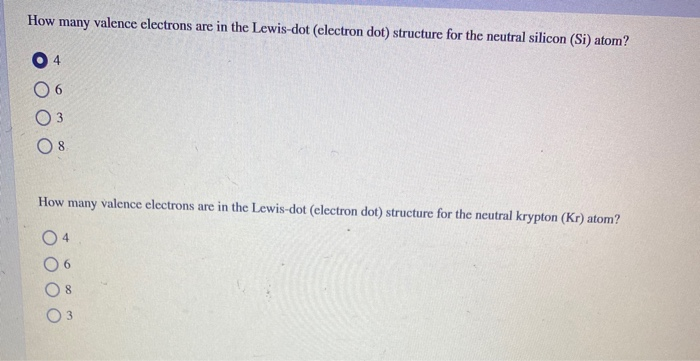
We review their content and use your feedback to keep the quality high. 100% (30 ratings) Transcribed image text: How many valence electrons are in the Lewis-dot (electron dot) structure for the neutral silicon (Si) atom? How many valence electrons are in the Lewis-dot (electron dot) structure for the neutral krypton (Kr) atom? 03 O 6.
For atoms with partially filled d or f subshells, these electrons are typically omitted from Lewis electron dot diagrams. For example, the electron dot diagram for iron (valence shell configuration 4s 2 3d 6) is as follows:. Elements in the same column of the periodic table have similar Lewis electron dot diagrams because they have the same valence shell electron configuration.
Question: Chapter 9 Chemical Bonds 9.1 Draw The Lewis Electron Dot Diagram For Each Element. A Strontium B. Silicon C. Krypton D. Sulfur 9.2 Draw The Lewis Electron Dot Diagram For Each Ion. A. In Lin B. Br Br: 9.3 How Many Electrons Does A Pb Atom Have To Lose To Have A Complete Octet In Its Valence Shell? 9.4 How Many Electrons Does An N Atom Have To Gain To ...
Feb 7, 2012 — Krypton has a full outer shell of eight electrons, so it would be eight dots, either around the outer circle if you're doing a full circle ...
KrF2 Lewis Structure, Hybridization, Molecular Geometry, and Polarity. KrF2 or Krypton difluoride is made up of Krypton and Fluorine and is one the first compounds of Krypton. It is a colorless solid which is highly volatile and thermally unstable. Although it decomposes at room temperature, it can be stored indefinitely at -78 degrees Celsius.
Exercises Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. Krypton difluoride, or KrF 2, has the Lewis structure of a krypton atom with 3 lone pairs, single bonded to two fluorine atoms, each also containing 3 lone pairs.
A step-by-step explanation of how to draw the Lewis dot structure for Kr (Krypton). I show you where Krypton is on the periodic table and how to determine h...
Krypton(Kr) is in Group 18 (sometimes called Group VIII or 8A or When you draw the Lewis structure for Krypton you'll put eight "dots" or.Aug 04, · Krypton difluoride, or KrF 2, has the Lewis structure of a krypton atom with 3 lone pairs, single bonded to two fluorine atoms, each also containing 3 lone pairs.
Lewis Dot Diagram (Measuring Matter) Lewis Dot Diagram for Krypton. Orbital Diagram . Orbital Diagram for Krypton . Quick Facts. Krypton is odorless and colorless. There are six stable isotopes of Krypton that occur in nature. Krypton is found in the Earth's atmosphere at about one part per million.
15 Electron Dot Diagram. Learn about electron dot diagram with free interactive flashcards. Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. Lewis Dot Structure for Krypton Atom (Kr) - YouTube from i.ytimg.com The only electrons shown…
Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
Krypton. Lewis (Dot) Diagram: Even atoms with more than. 20 electrons are easy.Krypton difluoride, or KrF 2, has the Lewis structure of a krypton atom with 3 lone pairs, single bonded to two fluorine atoms, each also containing 3 lone pairs. Krypton has 8 valence electrons, whereas fluorine contains 7 valence electrons.




















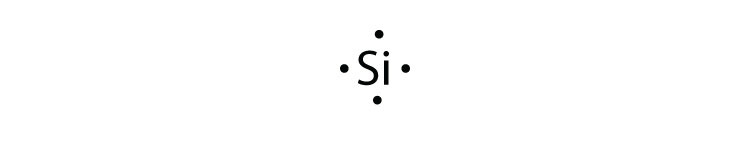





0 Response to "38 lewis dot diagram for krypton"
Post a Comment